Reporting of pre-analytical variables in RNA-focused blood plasma studies: a prerequisite for quality assessment and replication
Post-doctoral fellow Anneleen Decock presents her recent work on reporting pre-analytical variables in RNA-focused blood plasma studies.
Background: Blood-derived liquid biopsies are appealing biomaterials for various purposes in many medical disciplines as they offer a wide spectrum of analytes to be studied, including extracellular RNA (cell-free RNA; exRNA). The outcome of exRNA measurements is greatly influenced by pre-analytical variables, stressing the importance of disclosing pre-analytic variables in publications to enable adequate interpretation and comparison of research results. The aim of this review is to chart to what extent pre-analytics are currently documented in the exRNA literature to pinpoint potential shortcomings and to improve future reporting.
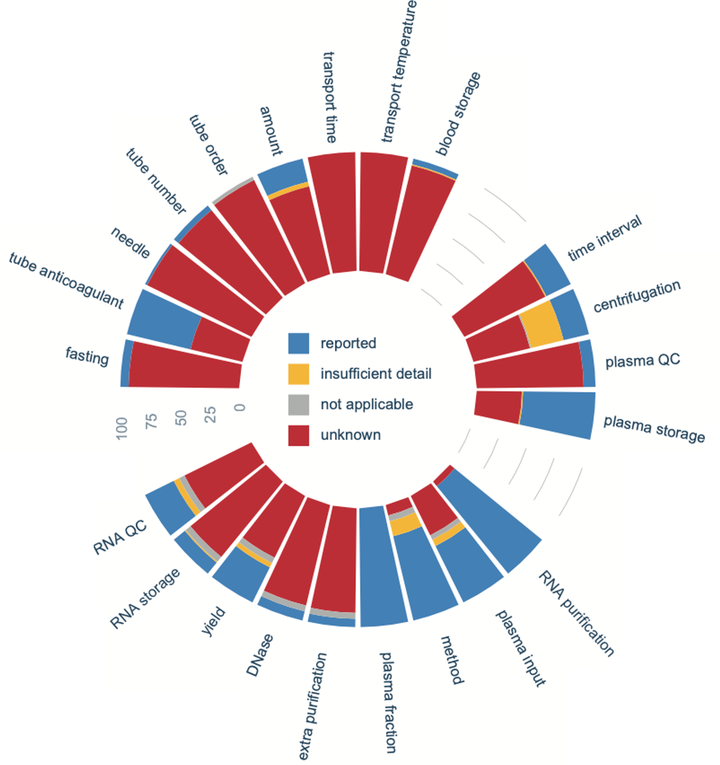
Methods: To this purpose, 100 publications on exRNA purified from blood plasma were reviewed for annotation of 22 encoded pre-analytical variables associated with blood collection, plasma preparation and RNA purification.
Results: In general, our results show that pre-analytics are poorly documented in literature, with only 6 out of 22 variables sufficiently described in more than half of the publications, including the blood collection tube anticoagulant, plasma storage temperature, RNA purification method and plasma input volume for RNA purification. Considering all variables, the percentage of variables reported in each publication ranges from only 1.0% to 13.6% (median of 7.0%).
Conclusions: Our results highlight the current lack of documenting pre-analytic variables in exRNA studies. We provide a reporting checklist that can be used for exRNA-based studies, hereby promoting the systematic reporting of relevant variables and enabling reliable interpretation, replication and standardization of future liquid biopsy studies.