A high-throughput CRISPRi screening platform to unravel functional long non-coding RNAs
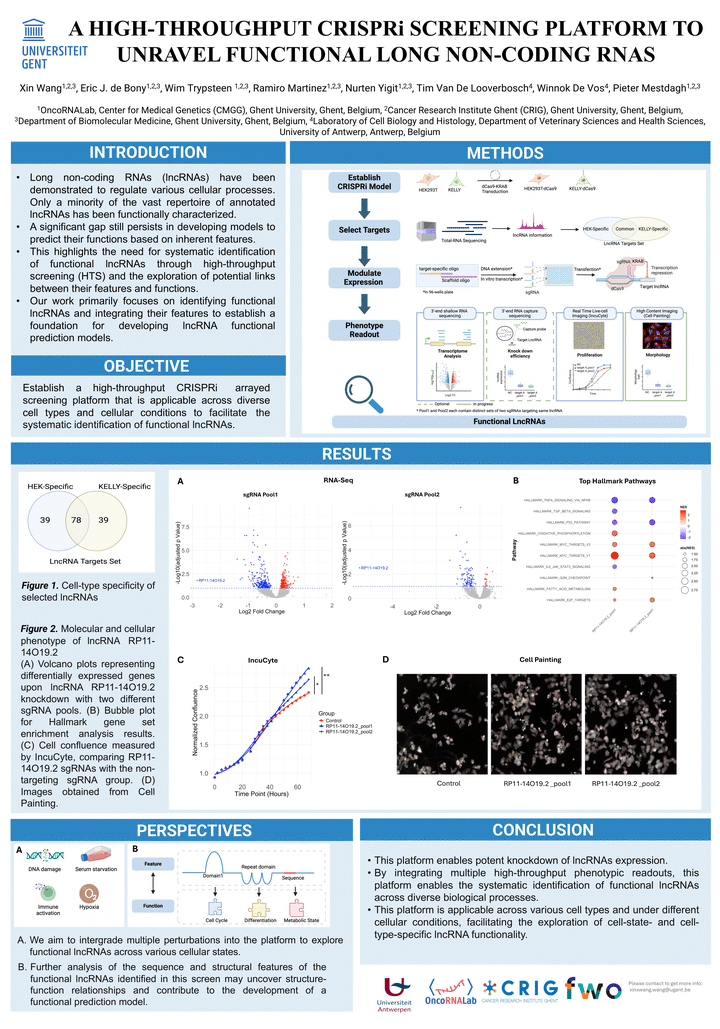
Long non-coding RNAs (lncRNAs) are a class of transcripts with lengths exceeding 200 nucleotides that do not encode proteins. Despite their crucial roles in cellular functions and biological processes, only a minority of the over 20,000 annotated lncRNAs have been functionally characterized. Here, we established a high-throughput, CRISPR-Interference (CRISPRi) arrayed screening plaKorm with serial cellular and molecular phenotyping to systematically characterize lncRNA functions. We reasoned that the integration of a comprehensive cellular and molecular phenotype can increase the probability of uncovering cellular functions and pathways controlled by lncRNA transcripts.
Based on the lncRNA expression data of two cell lines, we selected 156 lncRNAs that are either specifically expressed in one cell line or expressed in both cell lines. SgRNAs targeting the TSS region of these lncRNAs were produced through an optimized in vitro transcription (IVT) process and transfected in both cell lines stably expressing a dCas9 protein. Following the transfection of the sgRNAs, cells were analyzed by high-content microscopy (Cell Painting) and were then lysed for 3’- end shallow RNA-Seq. Preliminary results from this screen will be presented.
This plaKorm is applicable across different cell types and under varying cellular conditions, facilitating the exploration of cell-state- and cell-type-specific lncRNA functionality. Further analysis of the sequence and structural features of the functional lncRNAs identified through this screen may reveal structure–function relationships and could support the development of a functional prediction model.